Magnolia Bark GABAA Modulation Case Study
Electrophysiology and How is it Used to Understand Drug Mechanisms
Magnolia bark extract is a popular anti-anxiety herbal supplement. This extract contains two neurologically active compounds, Magnolol and Honokiol, both of which were found to be anxiolytic in humans and rodents. Their mechanism of action is to positively modulate the GABAA receptor, GABA’s primary target in the nervous system. When GABA binds to these receptors, an ion channel opens and Cl- is able to flow through and enter the neuron. Cl-, a negative ion, makes neurons more negative internally; therefore, less likely to fire an action potential. Enhancing the effects of these receptors will increase overall inhibition in the brain, resulting in less neurons firing and an anxiolytic, sedative effect.
This study, “The natural products magnolol and honokiol are positive allosteric modulators of both synaptic and extra-synaptic GABAA receptors” (link) aimed to further investigate the mechanisms of these compounds and see if their action is sub-unit specific. Here I’ll focus on the former, honing in on the researcher’s single cell voltage-clamp recordings that were used to measure GABAergic activity.
What is voltage-clamp recording?
Voltage-clamp is an electrophysiology technique commonly used in neuroscience research. It involves inserting a small glass electrode through the membrane and into a neuron, granting the electrode access to the inside of the neuron. This process is highly detailed in the methods of this paper under the section “Hippocampal slice recording”, but I’ll sum it up here in an attempt to make it more understandable.
Researchers can remove the brain from a rodent (rat here) and keep it alive in a cold artificial cerebrospinal fluid (aCSF) solution. aCSF contains everything needed to keep neurons alive; a mixture of electrolytes (Na+, K+, Ca2+, Cl-, MG2+) and glucose. It’s essentially just salty sugar water. The brain is then cut into 300 micrometer thin slices, and stored in room temperature aCSF that is being bubbled with oxygen. The goal here is to keep neurons alive so they can be recorded from.
When it’s time to record, brain slices are taken and placed into warm, oxygenated aCSF in an electrophysiology rig. This rig contains everything needed to perform these recording experiments including a well to hold the brain slice, various pipette and electrode holders, recording equipment hooked up to computers, etc.
In short, brain slices are collected, and the tissue can be kept alive to run experiments on.
Slices are taken one at a time, and the researchers can ‘patch’ into individual neurons with small glass electrodes. This process involves pressing the pipette against the cell membrane and ejecting a bit of pressure outwards to break through, leaving them with a recording electrode located inside the neuron.
This electrode has its own internal solution that will enter the neuron upon patching. This internal solution contains substances that block different ion channels (Cs, cesium, blocks K+ leak channels and QX-314 blocks voltage-gated Na+ channels) and enters the neuron. Blocking these ion channels removes any sources of ion flow that aren’t neurotransmitter receptors and inhibits action potentials. For this experiment, NMDA and AMPA glutamate receptors are also blocked by 2 drugs in the extracellular solution.
This setup makes the pathed neuron an ideal environment to record GABA currents because it has no way to change its internal voltage other than GABA-gated ion channels (GABAA receptors), or the electrode inside that allows the researchers to control and measure current in and out of it.
This is where the term “voltage-clamp” comes in. This electrode is hooked up to a series of amplifiers and a computer which allows the researchers to ‘clamp’ the voltage of the neuron at a desired voltage for the experiment. The voltage changes based on what they are recording and the established concentrations of ions (specifically chloride). The specifics of this aren't as important as knowing what is being recorded.
What's being recorded is the current that's being injected into the neuron by the electrode. Clamping the neuron at a specific voltage requires a computer to read changes in internal voltage (caused by ion flow in or out) and correct for that by injecting current.
For example, when GABAA receptors open, Cl- enters the neuron making it more negative. The electrode reads this change and injects an equal positive current back into the neuron to negate this negative change. The current injected into the neuron is the read out they get, since it is equal to the current caused by the opening of the receptors. That’s how this data is collected, and the neuron remains at the desired internal voltage.
Two different types of recordings were done for this paper; one measuring inhibitory postsynaptic currents (IPSCs) in response to a GABA neuron firing an action potential and releasing a ton of GABA onto one synapse, and the other which records miniature IPSCs (mIPSCs) which are the result of spontaneous, random vesicle releases not caused by an action potential. The former gives us a better understanding of the active compounds' effects on GABAA, so I’ll cover that here and touch on the latter at the end.
Measuring GABAA mediated IPSCs
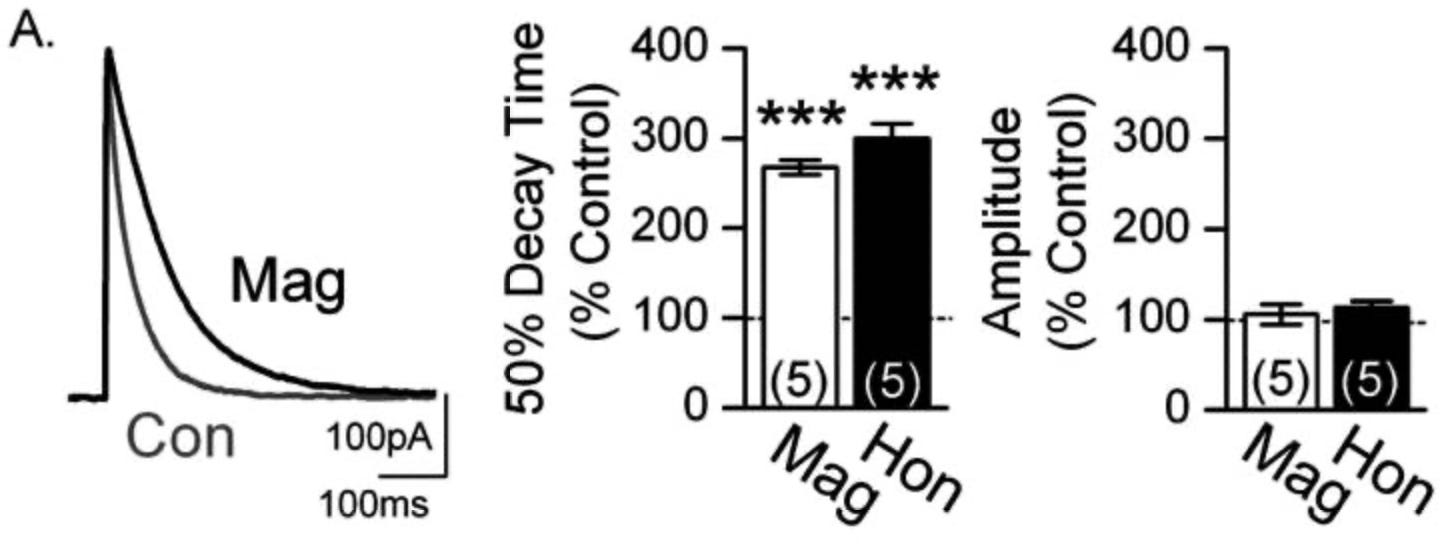
For this experiment, the neuron to be recorded was set up as described above. To get IPSCs though, a GABA neuron needs to be stimulated to fire an action potential to release GABA onto the neuron they are recording. To do so, they inserted an electrode into a GABAergic neuron, which they could then inject current into and cause it to fire an action potential on demand, releasing GABA. So, the researchers have one electrode to stimulate GABA release, and another to record changes in voltage (where the data comes from). This was done without the active components of Magnolia bark, then with them added to the bath. The results are shown in the figure below.
On the left, we have the average trace of current in response to these evoked IPSCs, control in grey and magnolol added in black. This is the trace of current that enters the neuron in response to GABA binding to GABAA receptors. The graphs to its right are showing the same things we can see in the trace, just quantified.
What does this figure tell us?
The amplitude of the IPSC is unchanged with the addition of magnolol and honokiol.This is shown most clearly in the far right graph. Amplitude of the trace is shown as a percentage of the control trace. Both substances show no change in the peak amplitude. These results indicate that neither magnolol or honokiol allow a greater flow of ions through at peak. The initial response is the same with or without these compounds
The decay time of the IPSC is greatly increased by the presence of these 2 substances. This is clear from both the trace and the first graph. This indicates that the GABAA receptors are open for longer when activated by GABA in the presence of magnolol and honokiol, or they're closing is delayed on average, dragging out the decay of current. With just GABA (control) the channels open instantly and close very fast (sharp decline in the trace), but in the presence of these 2 compounds the tail is elongated, indicating that the channels aren't closing as fast.
From these results, you can conclude that the action of these compounds at the synapse is to slow the deactivation rate of GABAA receptors without increasing their peak response. (Without increasing peak response is important to keep in mind, as that is not the case extra-synaptically)
How do they know these currents were only mediated by GABAA receptors?
They added bicuculline, a GABAA antagonist, to the bath after the experiment and found that these currents were completely blocked. If all current recorded is stopped by simply adding a GABAA antagonist, you can assume that all of the recorded current is coming from GABAA receptors.
mIPSC Recordings
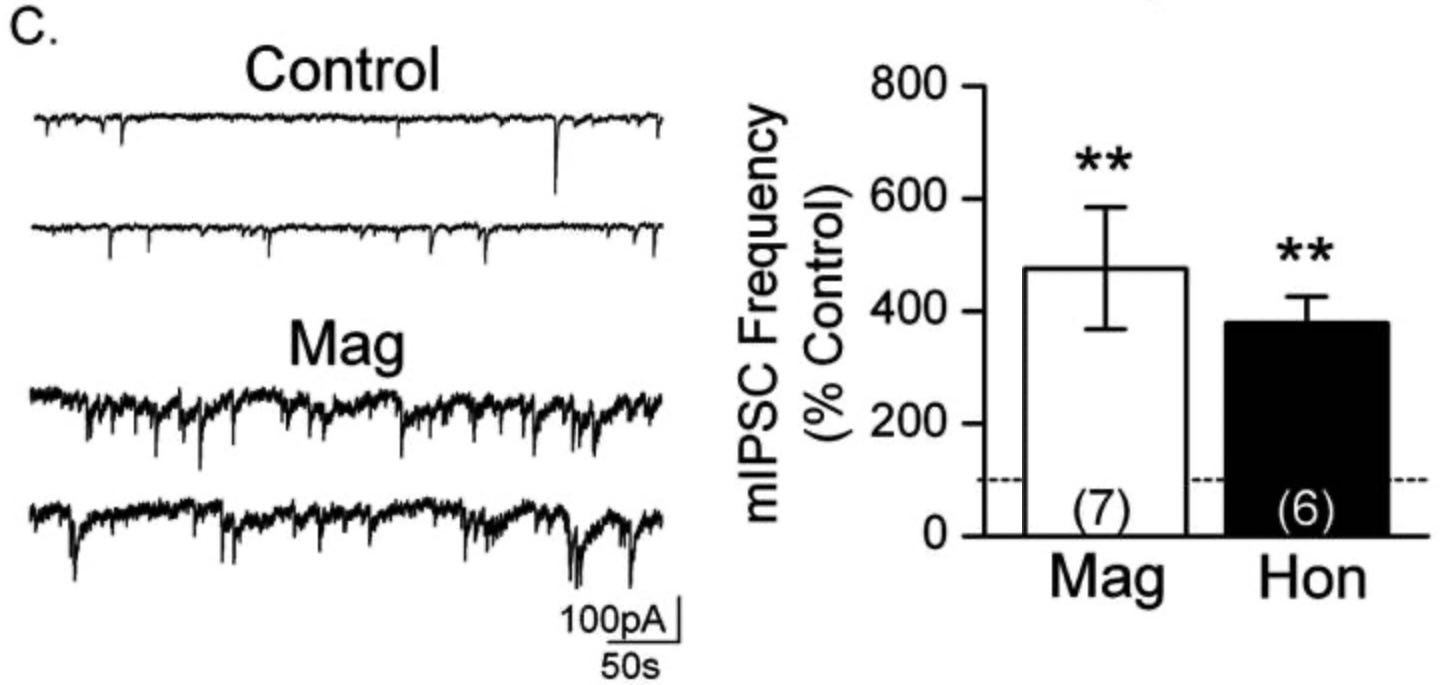
When recording miniature IPSCs, TTX is added to the bath containing the brain slice. TTX is a toxin that blocks voltage-gated sodium channels of all neurons in the slice, effectively inhibiting any action potentials from taking place. This allows you to record signals only from random vesicular releases, just a spontaneous single vesicle full of neurotransmitters being released not in response to an action potential. This provides insight into the presynaptic inner workings, since if frequency of release is increased, something must be causing that to occur.
This is what we see in Figure 1c:
The findings show a massive increase in mIPSC frequency, but no change in their amplitude. This is significant because it shows these compounds may have the ability to increase GABA release from the presynaptic neuron. There’s no way to derive a possible mechanism from these results, but an average increase of 400% is staggering and worth noting.
Figure 1B provides some interesting data as well, showing us that magnolol and honokiol can potentiate current coming from extrasynaptic GABAA receptors (which have a different subunit makeup and function). This was recorded in a similar fashion to the data from 1C, but I want to cover this in a separate post because we can dive into the importance of subunit composition and tonic vs phasic current. Phasic being activity at the synapse mostly driven by action potentials, and tonic being current that is always there caused by neurotransmitter ‘spillover’ from synapses.
Conclusion
Based on the findings of this paper and those prior, the two main psychoactive compounds in magnolia bark extract potentiate the action of GABA by enhancing activity of the GABAA receptor. That is what makes this supplement a potent anxiolytic. This action seems to be different depending on the subunit composition of the receptor, but we’ll explore that in an upcoming post as it would be way too much to cover here.
Thank you for reading!
Source:
Alexeev M, Grosenbaugh DK, Mott DD, Fisher JL. The natural products magnolol and honokiol are positive allosteric modulators of both synaptic and extra-synaptic GABA(A) receptors. Neuropharmacology. 2012 Jun;62(8):2507-14. doi: 10.1016/j.neuropharm.2012.03.002. Epub 2012 Mar 12. PMID: 22445602; PMCID: PMC3652012.


Hey BTN, love the blog. I got the intro email but the link to download the ebook pdf was broken. How can I still get the PDF?